Multiple Choice
If shaded and unshaded spheres represent atoms of different elements,as shown in drawing (1) ,which drawings (2) -(4) represent the law of multiple proportions? 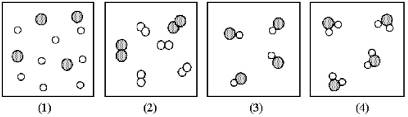
A) only drawings (2) and (3)
B) only drawings (2) and (4)
C) only drawings (3) and (4)
D) drawings (2) ,(3) ,and (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many electrons are in a neutral
Q8: Rb<sub>2</sub>S is named:<br>A)rubidium disulfide<br>B)rubidium sulfide<br>C)rubidium(II)sulfide<br>D)rubidium sulfur
Q20: Most of the alpha particles directed at
Q94: Which process decreases the neutron/proton ratio?<br>A)alpha emission<br>B)beta
Q114: . <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=". -If
Q211: What is the chemical symbol for an
Q243: The number of nucleons in a <img
Q247: Which of the following figures represents <img
Q249: If shaded and unshaded spheres represent atoms
Q253: Which of the compounds CH<sub>4</sub>,SrCl<sub>2</sub>,Cr(NO<sub>3</sub>)<sub>3</sub>,XeF<sub>2</sub> are expected