Multiple Choice
Which of the following figures represents 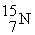
? Unshaded spheres represent neutrons and shaded spheres represent protons. 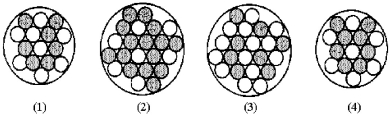
A) figure (1)
B) figure (2)
C) figure (3)
D) figure (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: How many electrons are in a neutral
Q8: Rb<sub>2</sub>S is named:<br>A)rubidium disulfide<br>B)rubidium sulfide<br>C)rubidium(II)sulfide<br>D)rubidium sulfur
Q20: Most of the alpha particles directed at
Q94: Which process decreases the neutron/proton ratio?<br>A)alpha emission<br>B)beta
Q114: . <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=". -If
Q123: Which of the following elements would you
Q211: What is the chemical symbol for an
Q243: The number of nucleons in a <img
Q248: If shaded and unshaded spheres represent atoms
Q249: If shaded and unshaded spheres represent atoms