Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 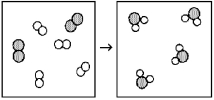
A) A + B → AB
B) 4A + 2B → 4AB
C) A2 + B2 → A2B
D) 2A2 + B2 → 2A2B
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q32: How many moles are there in 3.00
Q56: What is the identity of substance X
Q86: Balance the chemical equation given below,and determine
Q89: How many mL of a 0.175 M
Q92: The following diagram represents the reaction of
Q94: 7.0 g of nitrogen is reacted with
Q95: Calcium phosphate reacts with sulfuric acid to
Q96: If the reaction of phosphate ion with
Q107: When the reaction C<sub>4</sub>H<sub>10</sub> + O<sub>2</sub> →
Q144: Given the chemical equation: N<sub>2</sub> + 3