Multiple Choice
What is the balanced chemical equation for the reaction of element A (unshaded spheres) with element B (shaded spheres) as represented below? 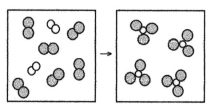
A) A + B → AB
B) A + 3B → 2AB
C) A2 + B2 → AB3
D) A2 + 3B2 → 2AB3
Correct Answer:

Verified
Correct Answer:
Verified
Q14: A student dissolved 4.00 g of Co(NO<sub>3</sub>)<sub>2</sub>
Q23: What is the molarity of a solution
Q48: Given the chemical equation: N<sub>2</sub> + 3
Q81: Which one of the following statements about
Q136: Which of the following has the smallest
Q164: If unshaded spheres represent nitrogen atoms and
Q165: Which one of the following contains 35%
Q167: Ascorbic acid,C<sub>6</sub>H<sub>8</sub>0<sub>6</sub>,can be represented by the molecular
Q170: How many moles of CuO can be
Q173: Calcium phosphate reacts with sulfuric acid to