Multiple Choice
Ascorbic acid,C6H806,can be represented by the molecular model shown below.If 1.00 mol of ascorbic acid is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 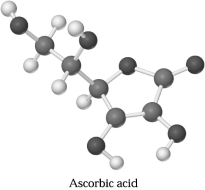
A) 3) 00 mol CO2 and 2.00 mol H2O
B) 6) 00 mol CO2 and 4.00 mol H2O
C) 6) 00 mol CO2 and 8.00 mol H2O
D) 12.0 mol CO2 and 10.0 mol H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q14: A student dissolved 4.00 g of Co(NO<sub>3</sub>)<sub>2</sub>
Q48: Given the chemical equation: N<sub>2</sub> + 3
Q81: Which one of the following statements about
Q90: The number of milliliters of 12.0 M
Q136: Which of the following has the smallest
Q163: Which represents one formula unit?<br>A)One H<br>B)One H<sub>2</sub><br>C)One
Q164: If unshaded spheres represent nitrogen atoms and
Q165: Which one of the following contains 35%
Q169: What is the balanced chemical equation for
Q170: How many moles of CuO can be