Multiple Choice
The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 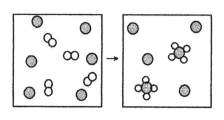
A) 2A2 + B → A4B;A2 is the limiting reactant.
B) 2A2 + B → A4B;B is the limiting reactant.
C) 4A2 + 6B → 2A4B;A2 is the limiting reactant.
D) 4A2 + 6B → 2A4B;B is the limiting reactant.
Correct Answer:

Verified
Correct Answer:
Verified
Q20: How many anions are there in 2.50
Q61: Combustion analysis of 1.200 g of an
Q86: What is the concentration of an AlCl<sub>3</sub>
Q96: Which of the following statements is false
Q108: The empirical formula of a compound that
Q133: What is the stoichiometric coefficient for oxygen
Q135: A hydrocarbon of unknown formula C<sub>x</sub>H<sub>y </sub>was
Q137: What mass of carbon dioxide,C O<sub>2</sub>,contains the
Q143: Box (1)represents 1.0 mL of a solution
Q162: When silver nitrate reacts with barium chloride,silver