Multiple Choice
Glucose,C6H1206,can be represented by the molecular model shown below.If 1.00 mol of glucose is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 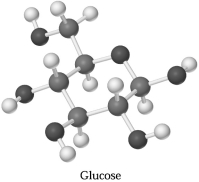
A) 1) 00 mol CO2 and 2.00 mol H2O
B) 6) 00 mol CO2 and 6.00 mol H2O
C) 6) 00 mol CO2 and 12.0 mol H2O
D) 12.0 mol CO2 and 12.0 mol H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q5: When the reaction C<sub>3</sub>H<sub>8</sub> + O<sub>2</sub> →
Q23: When methane,CH<sub>4</sub>,undergoes combustion with oxygen,the usual products
Q72: Box (1)represents 1.0 mL of a solution
Q97: The fundamental SI unit for measuring matter
Q156: What is the concentration of HCl in
Q195: Which substance is the limiting reactant when
Q196: A student dissolved 3.00 g of Co(NO<sub>3</sub>)<sub>2</sub>
Q198: Reaction of A (unshaded spheres)with B<sub>2</sub> (shaded
Q201: How many moles are in 1.50 g
Q203: How many grams of AgNO<sub>3</sub> are needed