Multiple Choice
Three different substances,A2X,A2Y,and A2Z,were dissolved in water with the following results.(Water molecules are omitted for clarity. ) Which of the substances is the strongest electrolyte,and which is the weakest? 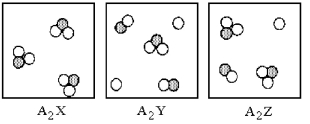
A) A2X is the strongest electrolyte and A2Y is the weakest electrolyte.
B) A2Y is the strongest electrolyte and A2X is the weakest electrolyte.
C) A2Y is the strongest electrolyte and A2Z is the weakest electrolyte.
D) A2Z is the strongest electrolyte and A2Y is the weakest electrolyte.
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Which species functions as the reducing agent
Q66: Water (H<sub>2</sub>O),methyl alcohol (CH<sub>3</sub>OH),ethyl alcohol (CH<sub>3</sub>CH<sub>2</sub>OH),ethylene glycol
Q68: Based on the balanced chemical equation shown
Q69: In the reaction 2 MnO<sub>4</sub><sup>-</sup>(aq)+ 10 Br<sup>-</sup>(aq)+
Q75: When dissolved in water,of HClO<sub>4,</sub> Ca(OH)<sub>2</sub>,KOH,HI,which are
Q78: Predict the products of a reaction between
Q98: Redox reactions occurring in acid are evident
Q122: Acetic acid (CH<sub>3</sub>CO<sub>2</sub>H),formic acid (HCO<sub>2</sub>H),hydrofluoric acid (HF),ammonia
Q128: Assume that the conductivity of a solution
Q146: Which of the following is not a