Multiple Choice
Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 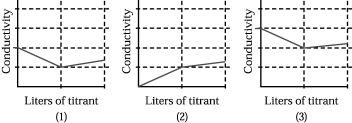
-Which of the above graphs corresponds to titration III?
A) graph (1)
B) graph (2)
C) graph (3)
D) None of the graphs
Correct Answer:

Verified
Correct Answer:
Verified
Q123: Coinage metals are metals that are not
Q124: In the following reaction,the reducing agent is
Q125: Which outcome corresponds to the combination of
Q126: What is the oxidation number of the
Q127: Which species functions as the reducing agent
Q129: Assume that the unshaded spheres in the
Q130: What is the oxidation number of the
Q131: Which outcome corresponds to the combination of
Q132: Which pair of compounds is soluble in
Q133: If the reaction of phosphate ion with