Multiple Choice
The following pictures represent aqueous solutions of three acids HA (A = X,Y,or Z) ,with water molecules omitted for clarity.Unshaded spheres represent hydrogen atoms or ions and gray spheres represent A atoms or ions.Which of the three is the strongest acid,and which is the weakest? 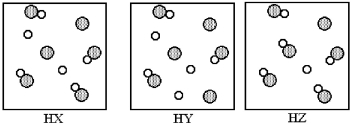
A) HX is the strongest acid and HY is the weakest acid.
B) HY is the strongest acid and HX is the weakest acid.
C) HY is the strongest acid and HZ is the weakest acid.
D) HZ is the strongest acid and HY is the weakest acid.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Write a balanced net ionic equation for
Q39: The balanced net ionic equation for the
Q75: The reaction shown below is classified as
Q106: The reaction HNO<sub>3</sub>(aq)+ KOH(aq)→ KNO<sub>3</sub>(aq)+ H<sub>2</sub>O(l)is best
Q125: In which compound is the oxidation state
Q126: Which species functions as the oxidizing agent
Q127: Based on the balanced chemical equation shown
Q130: According to the balanced equation shown below,4.00
Q133: Three different substances,A<sub>2</sub>X,A<sub>2</sub>Y,and A<sub>2</sub>Z,were dissolved in water
Q195: Assume that an aqueous solution of a