Multiple Choice
Two electromagnetic waves are represented below. 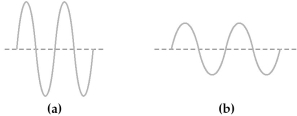
-Wave (b) has the
A) higher frequency and higher energy than wave (a) .
B) higher frequency and lower energy than wave (a) .
C) lower frequency and higher energy than wave (a) .
D) lower frequency and lower energy than wave (a) .
Correct Answer:

Verified
Correct Answer:
Verified
Q78: The spheres below represent atoms of Li,Be,B,and
Q79: Two electromagnetic waves are represented below. <img
Q80: Which of the following does not have
Q81: How many unpaired electrons are there in
Q82: An electron in a 4p orbital can
Q84: The element Ga has how many valence
Q85: Compared to Si ,Cl has a _
Q86: For the fourth-shell orbital shown below,what are
Q87: For the fourth-shell orbital shown below,what are
Q88: The symbol [Kr] represents<br>A)4s<sup>2</sup>4p<sup>6</sup>.<br>B)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>C)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>.<br>D)1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</sup>4s<sup>2</sup>4p<sup>6</sup>4d<sup>10</sup>.