Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 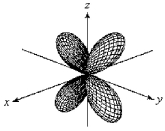
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q2: An oxygen molecule has a mass of
Q5: The number of orbitals having the quantum
Q8: Dentists employ light-cured materials to fill cavities.The
Q11: Which of the following have the same
Q15: For a multielectron atom,a 3s orbital lies
Q37: Of the following,which atom has the largest
Q82: What is the general valence-electron ground-state electron
Q96: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q111: Which of the following is not quantized?<br>A)the
Q143: Two electromagnetic waves are represented below. <img