Multiple Choice
Two electromagnetic waves are represented below. 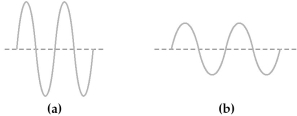
-Wave (a) has the
A) longer wavelength and higher energy than wave (b) .
B) longer wavelength and lower energy than wave (b) .
C) shorter wavelength and higher energy than wave (b) .
D) shorter wavelength and lower energy than wave (b) .
Correct Answer:

Verified
Correct Answer:
Verified
Q138: Which has the highest Z<sub>eff</sub> for its
Q139: Which element has the ground-state electron configuration
Q140: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q141: What is the frequency of a helium-neon
Q142: Which of the following is not true?<br>A)All
Q144: The Balmer-Rydberg equation can be extended to
Q145: What is the energy of a wavelength
Q146: Which of the following have the same
Q147: What is the general valence-electron ground-state electron
Q148: A radio station that broadcasts at 99.1