Multiple Choice
For the fourth-shell orbital shown below,what are the principal quantum number,n,and the angular momentum quantum number,l? 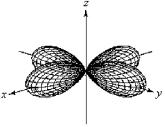
A) n = 4 and l = 0
B) n = 4 and l = 1
C) n = 4 and l = 2
D) n = 4 and l = 3
Correct Answer:

Verified
Correct Answer:
Verified
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q50: According to the Heisenberg uncertainty principle,<br>A)the position
Q68: The intensity of a beam of light
Q94: The average bond dissociation energy of a
Q132: The spheres below represent atoms of Sb,As,P,and
Q140: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q145: Atoms of which element,indicated by letter on
Q151: Which of the following have their valence
Q152: A neutral sulfur atom has how many
Q153: For a particular orbital,as one goes away