Multiple Choice
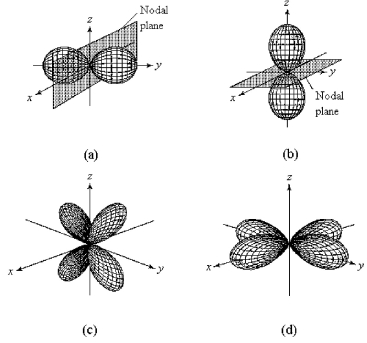
-Which of the above fourth-shell orbitals is a 4pz orbital?
A) orbital (a)
B) orbital (b)
C) orbital (c)
D) orbital (d)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q135: According to the Balmer-Rydberg equation,electromagnetic radiation with
Q136: How many h orbitals are allowed in
Q137: Which orbital-filling diagram violates the Pauli exclusion
Q138: Which has the highest Z<sub>eff</sub> for its
Q139: Which element has the ground-state electron configuration
Q141: What is the frequency of a helium-neon
Q142: Which of the following is not true?<br>A)All
Q143: Two electromagnetic waves are represented below. <img
Q144: The Balmer-Rydberg equation can be extended to
Q145: What is the energy of a wavelength