Multiple Choice
The spheres below represent atoms of Sb,As,P,and N (not necessarily in that order) . 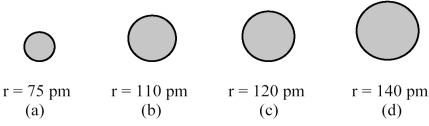
-Which one of these spheres represents an atom of As?
A) sphere (a)
B) sphere (b)
C) sphere (c)
D) sphere (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q115: The absorption of light of frequency 1.16
Q116: Arrange the following spectral regions in order
Q117: The spheres below represent atoms of Li,Be,B,and
Q118: Two electromagnetic waves are represented below. <img
Q119: What is the ground-state electron configuration of
Q121: Which orbital-filling diagram represents the ground state
Q122: The spheres below represent atoms of Li,Be,B,and
Q123: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q124: According to the Balmer-Rydberg equation,which transition results
Q125: What is the de Broglie wavelength of