Multiple Choice
Calculate the lattice energy for NaCl(s) using a Born-Haber cycle and the following information: 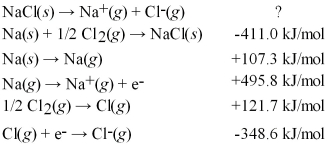
A) +34.8 kJ/mol
B) +690.3 kJ/mol
C) +787.2 kJ/mol
D) +1512 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Using shorthand notation,the ground-state electron configuration for
Q85: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q95: Which is not a chemical reaction of
Q128: To reach a noble gas electron configuration
Q129: Which alkali metal forms preferentially an oxide
Q130: Which of the following ionic compounds would
Q131: What is the name for the group
Q133: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt=" -Which element,indicated by
Q135: Using shorthand notation,the ground-state electron configuration for
Q136: The following four spheres represent a Na