Multiple Choice
Calculate the energy change for the formation of MgBr2(s) from its elements in their standard states: 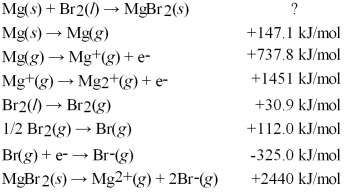
A) -150.8 kJ/mol
B) -286.0 kJ/mol
C) -499.2 kJ/mol
D) -5682 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The four spheres below represent K<sup>+</sup>,Ca<sup>2+</sup>,Cl<sup>-</sup>,and S<sup>2-</sup>,not
Q32: Which ion does not have a noble
Q63: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q72: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q78: Which alkaline earth metal reacts the most
Q85: Which contains both covalent bonds and ionic
Q86: Potassium reacts with oxygen to form a
Q87: Calculate the lattice energy for MgO(s)using a
Q88: What is the ground-state electron configuration of
Q128: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the