Multiple Choice
Calculate the electron affinity for the formation of the hydride ion from the following information: 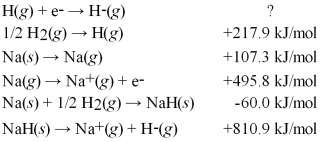
A) -50.1 kJ/mol
B) -70.1 kJ/mol
C) -816 kJ/mol
D) -1632 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which ionization process requires the most energy?<br>A)W(g)→
Q31: Of the following,which element has the highest
Q45: The following four spheres represent an Mg
Q48: The octet rule is most likely to
Q49: How many valence shell electrons does an
Q51: The ion Q<sup>2+</sup> contains 10 electrons.The identity
Q64: The tripositive ion with the electron configuration
Q122: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q124: The third-row element having a less negative
Q216: Each of the pictures (a)-(d)represents one of