Multiple Choice
The following four spheres represent an Mg atom,an Mg2+ ion,a S atom,and a S2- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 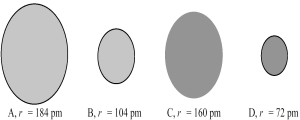
A) A → B + 2e⁻ and C → D + 2e⁻
B) A → B + 2e⁻ and D → C + 2e⁻
C) B → A + 2e⁻ and C → D + 2e⁻
D) B → A + 2e⁻ and D → C + 2e⁻
Correct Answer:

Verified
Correct Answer:
Verified
Q4: Which ionization process requires the most energy?<br>A)W(g)→
Q31: Of the following,which element has the highest
Q40: An incorrect statement about the alkaline earth
Q46: Calculate the electron affinity for the formation
Q48: The octet rule is most likely to
Q49: How many valence shell electrons does an
Q64: The tripositive ion with the electron configuration
Q122: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q124: The third-row element having a less negative
Q216: Each of the pictures (a)-(d)represents one of