Multiple Choice
Based on the indicated electronegativities,arrange the following in order of increasing ionic character: CsBr,LaBr3,PBr3,MgBr2. 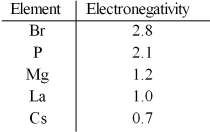
A) CsBr,LaBr3,MgBr2,PBr3
B) CsBr,MgBr2,PBr3,LaBr3
C) PBr3,LaBr3,MgBr2,CsBr
D) PBr3,MgBr2,LaBr3,CsBr
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Of BrF<sub>3 </sub>and PF<sub>3,</sub>the one with the
Q50: The electronegativities for the elements vary from
Q62: What geometric arrangement of charge clouds is
Q78: Of NH<sub>4</sub><sup>+</sup><sub> </sub>and NH<sub>4</sub><sup>-</sup><sub> </sub>the one with
Q158: What are the bond angles in the
Q159: What is the geometry around the central
Q160: What is the geometry around the central
Q162: Which drawing represents a σ<sup>*</sup> antibonding molecular
Q165: The following MO diagram is appropriate for
Q168: What are the bond angles in the