Multiple Choice
The following MO diagram is appropriate for Li2 and Be2.Based on this diagram, 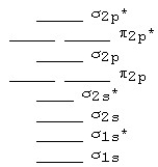
A) both are stable and diamagnetic.
B) Li2 is stable and diamagnetic,but Be2 is unstable.
C) Be2 is stable and diamagnetic,but Li2 is unstable.
D) Be2 is stable and paramagnetic,but Li2 is unstable.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Of BrF<sub>3 </sub>and PF<sub>3,</sub>the one with the
Q50: The electronegativities for the elements vary from
Q62: What geometric arrangement of charge clouds is
Q78: Of NH<sub>4</sub><sup>+</sup><sub> </sub>and NH<sub>4</sub><sup>-</sup><sub> </sub>the one with
Q88: The electronegativity for both sulfur and carbon
Q160: What is the geometry around the central
Q162: Which drawing represents a σ<sup>*</sup> antibonding molecular
Q163: Based on the indicated electronegativities,arrange the following
Q168: What are the bond angles in the
Q169: The orbital hybridization on the carbon atom