Multiple Choice
What is the geometry around the central atom in the following molecular model of XeF2? 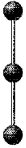
A) trigonal bipyramidal
B) seesaw
C) T-shaped
D) linear
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Classify bonds in As<sub>4</sub> as largely ionic,nonpolar
Q46: Compare the energies of molecular orbitals of
Q186: A molecular model of BeCl<sub>2</sub> is shown
Q188: Shown below is a model of SF<sub>6</sub>
Q189: Which molecule contains a triple bond?<br>A)F<sub>2</sub><br>B)O<sub>3</sub><br>C)HCN<br>D)H<sub>2</sub>CO
Q190: A chlorine atom in Cl<sub>2</sub> should have
Q193: The nitrogen-nitrogen bond in :N=N: has a
Q194: Which element can expand its valence shell
Q195: Shown below is a model of PF<sub>5</sub>
Q196: Using only the elements Ba,Cl,and P,give the