Multiple Choice
A molecular model of BeCl2 is shown below.Based on the best Lewis electron-dot structure for BeCl2 and formal charge considerations,what is the predicted Be-Cl bond order for each Be-Cl bond? 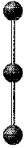
A) 1
B) 1) 33
C) 1) 5
D) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Ionic compounds consist of a single three-dimensional
Q46: Compare the energies of molecular orbitals of
Q61: What geometric arrangement of charge clouds is
Q64: Which type of bond produces a charge
Q96: The paramagnetism of O<sub>2</sub> is explained by<br>A)coordinate
Q185: Which drawing represents a σ bonding molecular
Q188: Shown below is a model of SF<sub>6</sub>
Q189: Which molecule contains a triple bond?<br>A)F<sub>2</sub><br>B)O<sub>3</sub><br>C)HCN<br>D)H<sub>2</sub>CO
Q190: A chlorine atom in Cl<sub>2</sub> should have
Q191: What is the geometry around the central