Multiple Choice
What is the geometry around the central atom in the following molecular model of BrF5? 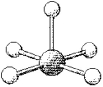
A) trigonal bipyramidal
B) octahedral
C) square pyramidal
D) square planar
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Of the following elements,which has the lowest
Q17: What are the F-Po-F bond angles in
Q28: Which atomic orbitals are involved in bonding
Q31: Which drawing represents the molecular orbital containing
Q36: Of the following elements,which has the lowest
Q38: How many lone pairs of electrons are
Q51: The VSEPR model predicts the H-O-H bond
Q65: Which of the following best describes ClF<sub>2</sub><sup>-</sup>?
Q70: If an electron is added to H<sub>2</sub>
Q79: Which molecule has the weakest bonds?<br>A)CF<sub>4</sub><br>B)CCl<sub>4</sub><br>C)CBr<sub>4</sub><br>D)CI<sub>4</sub>