Multiple Choice
Which atomic orbitals are involved in bonding and which as lone pair orbitals for N2H2? 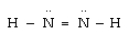
A) bonding: s on H,sp2 on N lone pair: p on N
B) bonding: sp2 on both H and N lone pair: p on N
C) bonding: s on H,p on N lone pair: sp2 on N
D) bonding: s on H,sp2 and p on N lone pair: sp2 on N
Correct Answer:

Verified
Correct Answer:
Verified
Q17: What are the F-Po-F bond angles in
Q23: Which electron dot structure for OCN<sup>-</sup> has
Q24: What are the bond angles in the
Q26: Based on VSEPR theory,which should have the
Q27: The Cl-Cl bond energy is 243 kJ/mol.Therefore
Q31: Which drawing represents the molecular orbital containing
Q33: What is the geometry around the central
Q41: What is geometry around the carbon atom
Q65: Which of the following best describes ClF<sub>2</sub><sup>-</sup>?
Q70: If an electron is added to H<sub>2</sub>