Multiple Choice
What are the bond angles in the following molecular model of NH4+? 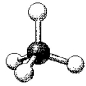
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: What is the geometry around the central
Q22: Classify bonds in CaO as largely ionic,nonpolar
Q23: Which electron dot structure for OCN<sup>-</sup> has
Q26: Based on VSEPR theory,which should have the
Q27: The Cl-Cl bond energy is 243 kJ/mol.Therefore
Q28: Which atomic orbitals are involved in bonding
Q41: What is geometry around the carbon atom
Q45: Which statement concerning any homonuclear diatomic molecule
Q46: Arrange the following in order of increasing
Q70: If an electron is added to H<sub>2</sub>