Multiple Choice
Which substance in each of the following pairs is expected to have the larger dispersion forces? 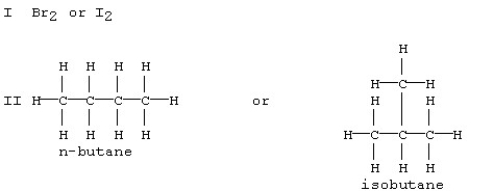
A) Br2 in set I and n-butane in set II
B) Br2 in set I and isobutane in set II
C) I2 in set I and n-butane in set II
D) I2 in set I and isobutane in set II
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Use the diagram below to answer the
Q22: The highest coordination number for spherical packing
Q68: O<sub>2</sub> and O<sub>3</sub> are _ of oxygen.<br>A)allotropes<br>B)isomers<br>C)isotopes<br>D)stereomers
Q86: Which drawing best represents hydrogen bonding in
Q90: When a liquid is heated at its
Q91: Identify the packing in the figure shown
Q97: Of C<sub>2</sub>H<sub>5</sub>OH and C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> the one expected
Q120: The plots below represent vapor pressure vs.temperature
Q123: The vapor pressure of a pure liquid
Q167: Which of the following should have the