Multiple Choice
The plots below represent vapor pressure vs.temperature curves for diethyl ether,ethanol,mercury,and water,not necessarily in that order. 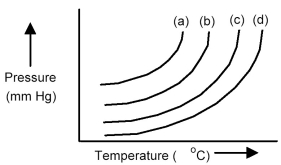
-Based on the relative strengths of the intermolecular forces of attraction of each substance,which is the most likely vapor pressure vs.temperature curve for ethanol?
A) curve (a)
B) curve (b)
C) curve (c)
D) curve (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q115: Iron crystallizes in a body-centered cubic cell
Q116: The critical temperature of a substance is
Q117: Bromine is one of only two elements
Q118: An ionic compound crystallizes in a unit
Q119: The phase diagram of a substance is
Q121: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -If figure (1)represents
Q122: What is the edge length of a
Q123: The vapor pressure of a pure liquid
Q124: A certain mineral,M<sub>x</sub>M'<sub>y</sub>A<sub>z</sub>,crystallizes in the cubic unit
Q125: Identify the packing in the figure shown