Multiple Choice
Which ion-dipole interaction results in the larger (more negative) hydration energy? 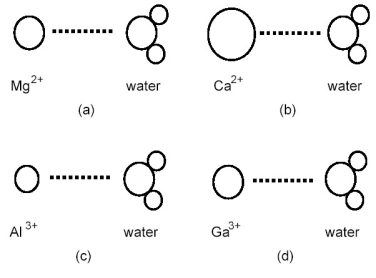
A) diagram (a)
B) diagram (b)
C) diagram (c)
D) diagram (d)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: The dose of amoxicillin given to a
Q12: KBr does not dissolve well in nonpolar
Q16: What molality of pentane is obtained by
Q19: To make a 2.00 m solution,one could
Q80: Sodium hydroxide is available commercially as a
Q83: A solution of 0.2113 g of water
Q154: Which of the following is not an
Q164: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q166: The vapor pressure of water at 25°C
Q177: Arrows in the energy diagram below represent