Multiple Choice
Drawings (1) and (2) show the equilibrium vapor pressures of two pure liquids.Which drawing (3) -(6) represents the equilibrium vapor pressure of a solution made by mixing equal moles of each liquid? 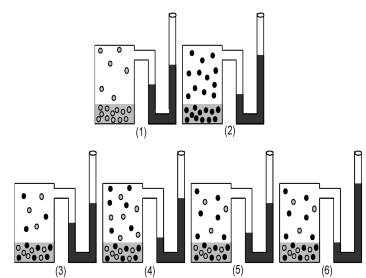
A) drawing (3)
B) drawing (4)
C) drawing (5)
D) drawing (6)
Correct Answer:

Verified
Correct Answer:
Verified
Q25: How will the osmotic pressure of an
Q68: For the process of dissolving a solid
Q71: Aqueous solutions of 30% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are
Q106: A solution is 2.25% by weight NaHCO<sub>3</sub>.How
Q109: A phase diagram of temperature versus composition
Q111: A solution of LiCl in water is
Q112: Which should be least soluble in water?<br>A)
Q113: A 1.30 M solution of CaCl<sub>2</sub> in
Q119: What is the molality of a glucose
Q160: Commercial cold packs often contain solid NH<sub>4</sub>NO<sub>3</sub>