Multiple Choice
At 80°C,pure liquid A has a vapor pressure of 700 mm Hg and pure liquid B has a vapor pressure of 940 mm Hg.What is XA for a solution of A and B with a normal boiling point of 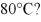
A) 0) 25
B) 0) 50
C) 0) 75
D) A solution of A and B cannot boil at 80°C.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Arrows in the energy diagram below represent
Q62: A solution is prepared by dissolving 17.75
Q127: How much water must be added to
Q131: Drawing (1)shows the equilibrium vapor pressure of
Q132: A 0.51 m aqueous solution of an
Q134: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q140: Iodine,I<sub>2</sub>(s),is more soluble in dichloromethane,CH<sub>2</sub>Cl<sub>2</sub>(l),than in water
Q170: Molarity is defined as _,whereas molality is
Q181: Red blood cells are placed into pure
Q191: Assuming that sea water is a 3.5