Multiple Choice
Write the equilibrium equation for the forward reaction:
2 CH4 (g) + 3 O2 (g) ⇌ 2 CO (g) + 4 H2O (g)
A) Kc = 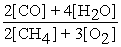
B) Kc = 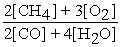
C) Kc = 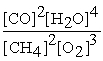
D) Kc = 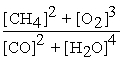
Correct Answer:

Verified
Correct Answer:
Verified
Q11: If additional SCN<sup>-</sup> is added to the
Q21: At an elevated temperature,K<sub>p</sub> = 4.2 ×
Q41: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q43: Phosphorus pentachloride decomposes to phosphorus trichloride and
Q71: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q74: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q80: Which of the following changes in reaction
Q113: The reaction A<sub>2</sub> + B<sub>2 </sub>⇌ 2AB
Q118: K<sub>p</sub> = 1.5 × 10<sup>3</sup> at 400°C
Q131: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298