Multiple Choice
The reaction A2 + B2 ⇌ 2AB has an equilibrium constant Kc = 1.8.The following pictures represent reaction mixtures that contain A2 molecules (shaded) and B2 molecules (unshaded) ,and AB molecules. 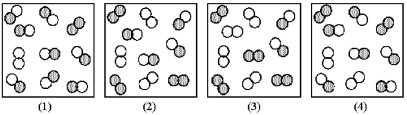
-Which nonequilibrium mixtures will react in the reverse direction to reach equilibrium?
A) reaction mixtures (1) and (2)
B) reaction mixtures (1) and (4)
C) reaction mixtures (2) and (3)
D) reaction mixtures (3) and (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q108: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500
Q110: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic at
Q111: In a reversible reaction,when the rate of
Q112: Picture (1)represents an equilibrium mixture of solid
Q114: Ammonium bromide is a crystalline solid that
Q115: A reaction in which reactants form products
Q116: Which of the following statements is false
Q117: The following picture represents the equilibrium state
Q118: K<sub>p</sub> = 1.5 × 10<sup>3</sup> at 400°C