Multiple Choice
Write the equilibrium equation for the forward reaction:
2 CH4(g) + 3 O2(g) ⇌ 2 CO(g) + 4 H2O(g)
A) Kp = 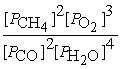
B) Kp= 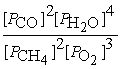
C) Kp = 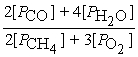
D) Kp = 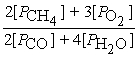
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which one of the following statements about
Q7: The following picture represents the equilibrium state
Q10: For a homogeneous equilibrium of gases,which of
Q13: What is the equilibrium equation for the
Q14: For the reaction: 4 HCl(g)+ O<sub>2</sub>(g)⇌ 2
Q16: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q44: If K<sub>c</sub> is the equilibrium constant for
Q81: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)=
Q81: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)=
Q134: Consider the reaction A + B ⇌