Multiple Choice
Consider the reaction A + B ⇌ 2 AB.The vessel on the right contains an equilibrium mixture of A atoms (shaded spheres) ,B atoms (unshaded spheres) ,and AB molecules. 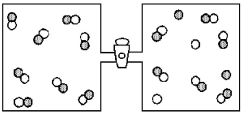
-If the barrier between the two vessels is removed and the contents of the two vessels are allowed to mix,what will be observed?
A) The reaction will go in the forward direction decreasing the number of A atoms and B atoms and increasing the number of AB molecules.
B) The reaction will go in the forward direction increasing the number of A atoms and B atoms and decreasing the number of AB molecules.
C) The reaction will go in the reverse direction decreasing the number of A atoms and B atoms and increasing the number of AB molecules.
D) The reaction will go in the reverse direction increasing the number of A atoms and B atoms and decreasing the number of AB molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q130: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q131: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q133: What is the equilibrium equation for the
Q135: Picture (1)represents an equilibrium mixture of solid
Q136: For the reaction: 4 HCl(g)+ O<sub>2</sub>(g)⇌ 2
Q137: If K<sub>c</sub> equals 0.110 at 25°C for
Q138: For the reaction H<sub>2</sub>(g)+ S(s)⇌ H<sub>2</sub>S(g),if the
Q139: For the reaction shown below,which change in