Multiple Choice
The following pictures represent mixtures of A2B4 molecules and AB2 molecules,which interconvert according to the equation A2B4 ⇌ 2 AB2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 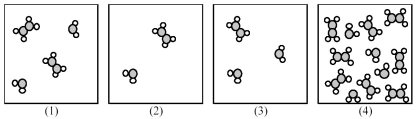
A) mixture (2)
B) mixture (3)
C) mixture (4)
D) None of the other mixtures are at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: At a certain temperature,nitrogen and hydrogen react
Q26: The enthalpy for the following reaction is
Q28: At 1000 K,K<sub>p</sub> = 19.9 for the
Q32: For the reaction shown below,which change in
Q57: Which one of the following statements does
Q67: Picture (1)represents the equilibrium mixture for the
Q88: Consider the interconversion of A molecules (shaded
Q90: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q146: A 1.50 L vessel contains an equilibrium
Q169: At an elevated temperature,K<sub>p</sub> = 0.19 for