Multiple Choice
BF3 and NH3 undergo a Lewis acid-base reaction forming an adduct.Which picture below correctly represents the curved arrow notation for the initial Lewis acid-Lewis base interaction in this reaction;what is the Lewis acid and the Lewis base? 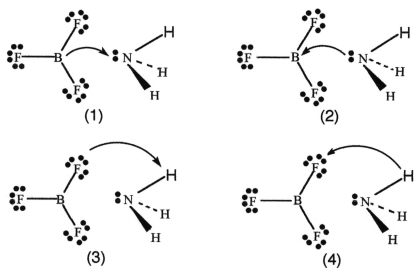
A) Picture (1) is correct;NH3 is the Lewis acid and BF3 is the Lewis base.
B) Picture (2) is correct;BF3 is the Lewis acid and NH3 is the Lewis base.
C) Picture (3) is correct;NH3 is the Lewis acid and BF3 is the Lewis base.
D) Picture (4) is correct;BF3 is the Lewis acid and NH3 is the Lewis base.
Correct Answer:

Verified
Correct Answer:
Verified
Q53: In order for the reaction A<sup>-</sup> +
Q95: Undersea flora prefer a maximum concentration of
Q145: Calculate the concentration of bicarbonate ion,HCO<sub>3</sub><sup>-</sup>,in a
Q185: What is the pH of a solution
Q188: Normal rainfall has a concentration of OH<sup>-</sup>
Q190: A 0.10 M KNO<sub>2</sub> solution will have
Q191: Which one of the following salts,when dissolved
Q192: A solution with a hydrogen ion concentration
Q192: In the following reaction the unshaded spheres
Q193: Potassium hydrogen phthalate (molar mass = 204.2