Multiple Choice
What is the molar solubility of CaF2 in 0.10 M NaF solution at 25°C? The Ksp for CaF2 is 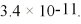
A) 8) 5 × 10-10 M
B) 3) 4 × 10-10 M
C) 3) 4 × 10-9 M
D) 2) 0 × 10-4 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: What is the pH of a buffer
Q127: What is the common ion in a
Q131: What is the approximate value of the
Q132: When 50 mL of 0.10 M NH<sub>4</sub>Cl
Q133: What is the equilibrium constant expression for
Q134: The following plot shows a titration curve
Q135: What is the chromium ion concentration for
Q143: What volume of 5.00 × 10<sup>-3</sup> M
Q166: What is the pH at the first
Q173: What is the approximate pH at the