Multiple Choice
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A+ with NaOH.Which point a-d represents the isoelectric point? 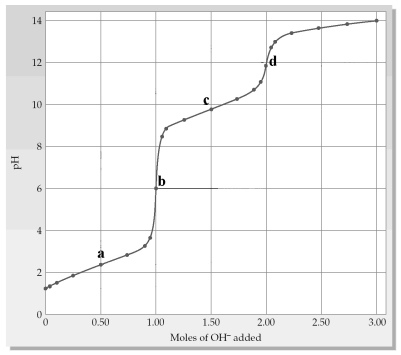
A) point a
B) point b
C) point c
D) point d
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: What is the pH of a buffer
Q111: Which of the following reactions are not
Q130: What is the molar solubility of CaF<sub>2</sub>
Q131: What is the approximate value of the
Q132: When 50 mL of 0.10 M NH<sub>4</sub>Cl
Q133: What is the equilibrium constant expression for
Q135: What is the chromium ion concentration for
Q136: When equal molar amounts of the following
Q137: What is the [CH<sub>3</sub>CO<sub>2</sub>-]/[CH<sub>3</sub>CO<sub>2</sub>H] ratio necessary to
Q139: What is the most soluble salt of