Multiple Choice
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN) 2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN) 2- is 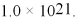
A) 0) 050 M
B) 0) 10 M
C) 0) 20 M
D) 0) 40 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Precipitation of an ionic compound will occur
Q7: What is the pH of a buffer
Q8: In which of the following solutions would
Q11: The balanced net ionic equation for the
Q13: Which of the following combinations of chemicals
Q15: The half equivalence point in the titration
Q32: A buffer solution is prepared by dissolving
Q86: The following pictures represent solutions that contain
Q93: What volume of 0.100 M NaOH is
Q170: The pH of a 0.150 M formic