Multiple Choice
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 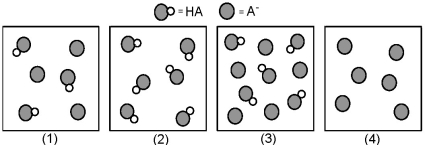
-Which solution has the greatest buffer capacity?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q81: What is the approximate pH at the
Q82: What is the approximate pH at the
Q83: Calculate the solubility (in g/L)of calcium fluoride
Q84: 0.10 M potassium chromate is slowly added
Q85: What is the pH of a buffered
Q87: What is the least soluble salt of
Q88: What is the molar solubility of lead(II)chromate
Q89: The solution formed upon adding 80.00 mL
Q90: What is the pH of the resulting
Q91: What is the pH of a solution