Multiple Choice
Which metal sulfides can be precipitated from a solution that is 0.01 M in Mn2+,Zn2+,Pb2+ and Cu2+ and 0.10 M in H2S at a pH of 1.0? 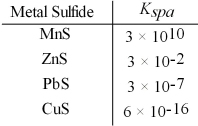
A) MnS
B) CuS
C) PbS,CuS
D) ZnS,PbS,CuS
Correct Answer:

Verified
Correct Answer:
Verified
Q50: Use the graphs below to answer the
Q52: The following plot shows two titration curves,each
Q59: The following plot shows a titration curve
Q71: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If
Q75: The following pictures represent solutions that contain
Q96: The neutralization constant K<sub>n</sub> for the neutralization
Q101: Silver oxalate,Ag<sub>2</sub>C<sub>2</sub>O<sub>4</sub>,has a molar solubility = 1.1
Q155: Which metal ions can be precipitated out
Q158: What is the pH of a solution
Q162: The dissociation equilibrium constants for the protonated