Multiple Choice
The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 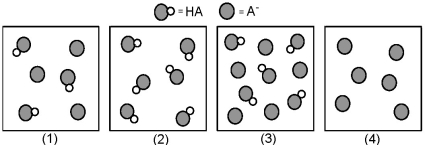
-For which solution(s) is pH = pKa?
A) only solution (1)
B) only solution (2)
C) only solution (3)
D) solutions (1) and (3)
Correct Answer:

Verified
Correct Answer:
Verified
Q172: Which of the following metal hydroxides are
Q173: What is the approximate pH at the
Q174: Which of the following titrations result in
Q175: The following plot shows a titration curve
Q176: A solution may contain the following ions
Q178: What is the K<sub>a</sub> of the amino
Q179: The following pictures represent solutions of AgCl,which
Q180: The following pictures represent solutions at various
Q181: What is the pH of a solution
Q182: The following pictures represent solutions at various