Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 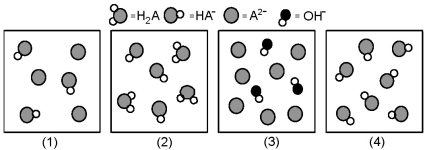
-Which picture represents the system with the lowest pH?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q68: What is the molar solubility of Mg(OH)<sub>2</sub>
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q70: The following plot shows a titration curve
Q71: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If
Q72: When 70 mL of 0.18 M NH<sub>4</sub>Cl
Q74: What is the most soluble salt of
Q75: The following pictures represent solutions that contain
Q76: The following pictures represent solutions that contain
Q78: What is the pH of a solution
Q157: The following pictures represent solutions that contain