Multiple Choice
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH. 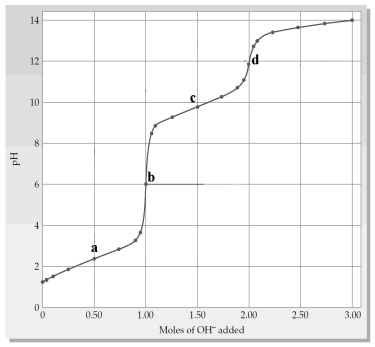
-Which point a-d represents the HX-/X2- buffer region?
A) point a
B) point b
C) point c
D) point d
Correct Answer:

Verified
Correct Answer:
Verified
Q65: What is the equilibrium constant expression for
Q66: Use the graphs below to answer the
Q67: Consider a buffered solution consisting of H<sub>2</sub>CO<sub>3</sub>
Q68: What is the molar solubility of Mg(OH)<sub>2</sub>
Q69: Oxalic acid,H<sub>2</sub>C<sub>2</sub>O<sub>4</sub> has acid dissociation constants K<sub>a1</sub>
Q71: CaF<sub>2</sub> has K<sub>sp</sub> = 3.5 × 10<sup>-11</sup>.If
Q72: When 70 mL of 0.18 M NH<sub>4</sub>Cl
Q73: The following pictures represent solutions at various
Q74: What is the most soluble salt of
Q75: The following pictures represent solutions that contain