Multiple Choice
Calculate the equilibrium constant,K,at 25°C for the galvanic cell reaction shown below: 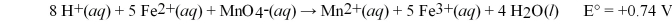
A) 3) 2 × 10-63
B) 3) 2 × 10-13
C) 3) 2 × 1012
D) 3) 2 × 1062
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: For a particular cell based on the
Q60: The bright colors of anodized titanium are
Q62: The standard potential for the following galvanic
Q65: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4939/.jpg" alt=" -Use Table 17.1
Q89: Which of the following reactions is most
Q118: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -For the galvanic
Q143: The Hall-Heroult process for the production of
Q147: What is least easily oxidized?<br>A)Al<br>B)Fe<br>C)Mg<br>D)Zn
Q153: The iron content of foods can be
Q182: Which statement below is not true?<br>A)The cell