Multiple Choice
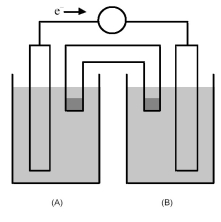
-For the galvanic cell shown above,in what direction should the anions and cations in half-cell A move?
A) The anions tend to move to the solid electrode and the cations into the salt bridge.
B) The anions tend to move into the salt bridge and the cations toward the solid electrode.
C) The anions tend to move toward the solid electrode and the cations should not move.
D) The cations tend to move toward the solid electrode and the anions should not move.
Correct Answer:

Verified
Correct Answer:
Verified
Q113: Consider the galvanic cell shown below. <img
Q114: In the relationship ΔG = -nFE°,what is
Q115: Calculate the cell potential at 25°C for
Q116: In a galvanic cell constructed from Pb(s)|
Q117: What is the balanced chemical equation for
Q119: Shown below are the reactions occurring in
Q120: A particular 12V battery is based on
Q121: Fuel cells<br>A)produce carbon dioxide and hydrogen.<br>B)emit sulfur
Q122: In the galvanic cell represented by the
Q123: Consider the following galvanic cell. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"