Multiple Choice
How many grams of zinc metal are required to produce 1.00 liter of hydrogen gas at STP according to the chemical equation shown below? 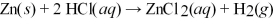
A) 0) 0343 g
B) 2) 92 g
C) 5) 84 g
D) 65.4 g
Correct Answer:

Verified
Correct Answer:
Verified
Q111: There are three different isotopes of hydrogen
Q150: Of O<sub>2</sub>,O<sub>2</sub><sup>-</sup>,and O<sub>2</sub><sup>2-</sup>,the oxygen-oxygen bond length is
Q151: In the following picture of an uncharged
Q153: Write a chemical equation representing the water
Q154: An example of an isotope effect is
Q156: What are the allotropes of oxygen?<br>A)O and
Q157: Water accounts for approximately two-thirds of the
Q158: How many grams of calcium hydride are
Q159: Which is not an isotope of hydrogen?<br>A)hydrogen-1<br>B)hydrogen-2<br>C)hydrogen-3<br>D)hydrogen-4
Q160: When 0.350 grams of anhydrous copper(II)sulfate is