Multiple Choice
In the following picture of an uncharged oxide,darkly-shaded spheres represent oxygen atoms or ions and lightly-shaded spheres represent atoms or ions of a second- or third-row element in its highest oxidation state.Identify the element represented by the lightly-shaded spheres. 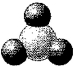
A) Al
B) C
C) N
D) S
Correct Answer:

Verified
Correct Answer:
Verified
Q111: There are three different isotopes of hydrogen
Q146: Look at the location of elements A,B,C,and
Q148: In the following picture of an oxide,darkly-shaded
Q149: What is not a characteristic of the
Q150: Of O<sub>2</sub>,O<sub>2</sub><sup>-</sup>,and O<sub>2</sub><sup>2-</sup>,the oxygen-oxygen bond length is
Q153: Write a chemical equation representing the water
Q154: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What group of
Q154: An example of an isotope effect is
Q155: How many grams of zinc metal are
Q156: What are the allotropes of oxygen?<br>A)O and